
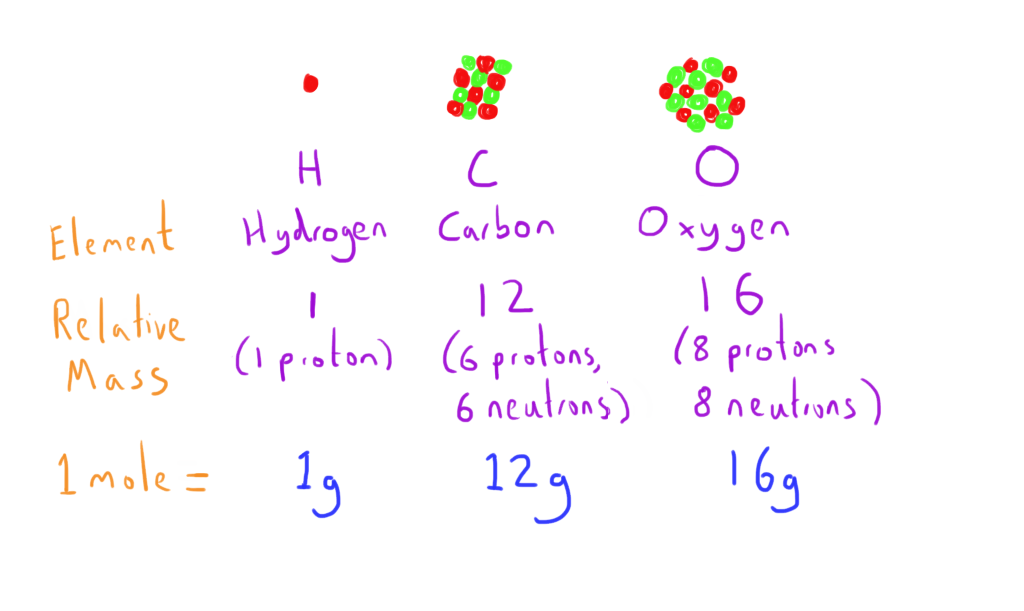
The division sign (/) implies “per,” and “1” is implied in the denominator. For example, the molar mass of Ba(OH) 2 requires the sum of 1 mass of Ba, 2 masses of O, and 2 masses of H: The molar mass of Ba(OH)2 requires the sum of 1 mass of Ba, 2 masses of O, and 2 masses of H: 1 Ba molar mass:īecause molar mass is defined as the mass for 1 mol of a substance, we can refer to molar mass as grams per mole (g/mol). Other atoms dont generally have round-number atomic masses for. In formulas with polyatomic ions in parentheses, the subscript outside the parentheses is applied to every atom inside the parentheses. By definition, an atom of carbon with six neutrons, carbon-12, has an atomic mass of 12 amu. The term mole is defined in that one mole of a substance with. adenosine (C 10H 13N 5O 4), a component of cell nuclei crucial for cell divisionīe careful when counting atoms. Also, important in this field is Avogadros number (NA) or Avogadros constant (6.0221 x 1023).The chemical formula of Nitric acid is HNO 3. Nitric acid is a strong acid which has a high oxidation power. This morning you drove at a speed of 95 km/h for 2. Molar mass is the sum of the masses of all the individual atoms present in a molecule or compound. The molar masses of N, H and C are 14.01 g/mol, 1.008 g/mol and 12.01 g/mol respectively. Calculate the mass of 4.8 mol of N2 (H C3). barium sulfate (BaSO 4), used to take X rays of the gastrointestional tract The atomic masses of N and O are 14.01 amu/atom and 16.00 amu/atom respectively.What is the mass of 1 mol of each substance? The truth is that atomic weights have changed as a function of time. Average atomic mass of any element is given by adding the multiplication of atomic masses of all the isotopes (stable isotopes) with their percentage abundances in the natural environment.\): Moles to Mass Conversion with Compounds Atomic weights found within a periodic table one might think are constant. We should remember that we can solve these types of questions simply by using the formula for average atomic mass. So, the abundance of two isotopes can be calculated using the average atomic mass formula as follows:
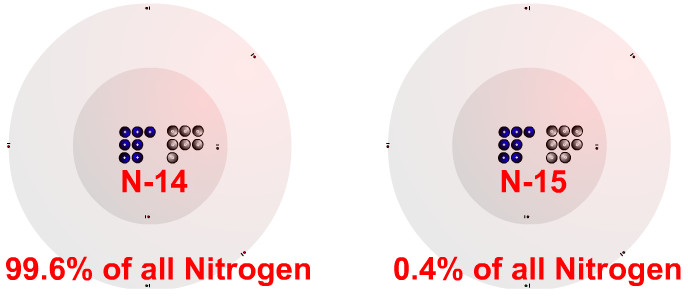
Atomic mass of any element depends upon its stable isotopes and their abundance in nature.įirst of all we will assume the abundance percentage of both the isotopes as follows: We know that Isotopes are basically defined as the two or more different atoms of the same element which have the same atomic numbers but different mass numbers. Name: Nitrogen Symbol: N Atomic Number: 7 Atomic Mass: 14.00674 amu Melting Point: -209.9 C (63.250008 K, -345.81998 F) Boiling Point: -195.8 C (77.35 K, -320.44 F) Number of Protons/Electrons: 7 Number of Neutrons: 7 Classification: Non-metal Crystal Structure: Hexagonal Density 293 K: 1. So, by using the given atomic masses of two isotopes, we can find out their percentage abundance in nature and then the ratio of atoms of both the isotopes in natural nitrogen. The atomic mass of an element is a weighted average of its isotopes in which the sum of the abundance of each isotope is equal to 1 or 100.
Isotopes are basically defined as the two or more different atoms of the same element which have the same atomic numbers but different mass numbers.

Hint: Atomic mass of any element depends upon its stable isotopes and their abundance in nature.


 0 kommentar(er)
0 kommentar(er)
